supergenus Bolitoglossa
Tom Devitt and David Wake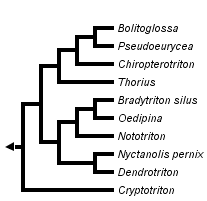

This tree diagram shows the relationships between several groups of organisms.
The root of the current tree connects the organisms featured in this tree to their containing group and the rest of the Tree of Life. The basal branching point in the tree represents the ancestor of the other groups in the tree. This ancestor diversified over time into several descendent subgroups, which are represented as internal nodes and terminal taxa to the right.

You can click on the root to travel down the Tree of Life all the way to the root of all Life, and you can click on the names of descendent subgroups to travel up the Tree of Life all the way to individual species.
For more information on ToL tree formatting, please see Interpreting the Tree or Classification. To learn more about phylogenetic trees, please visit our Phylogenetic Biology pages.
close boxIntroduction
The supergenus Bolitoglossa has undergone an extensive adaptive radiation in Neotropical middle America and South America (Wake and Lynch, 1976), containing about two-thirds of plethodontid species and 40% of all species of salamanders. Thirteen genera comprise the supergenus Bolitoglossa: Bolitoglossa, Bradytriton, Chiropterotriton, Cryptotriton, Dendrotriton, Ixalotriton, Lineatriton, Nototriton, Nyctanolis, Oedipina, Parvimolge, Pseudoeurycea, and Thorius. Parvimolge, Lineatriton and Ixalotriton are nested within the large genus Pseudoeurycea, pending revision of the latter. At present Pseudoeurycea sensu lato includes 51 species. The genus Bolitoglossa represents the largest and most widely distributed genus of salamander, with over 90 species, or about 17% of all recognized salamander species (AmphibiaWeb: Amphibian Facts).
Convergent and parallel evolutionary origins of webbing of the feet are observed in several genera, with the most extensive webbing found in arboreal and semiarboreal members of the genus Bolitoglossa (Wake and Lynch, 1976; Alberch, 1981; Wake, 1991; Parra-Olea et al., 2004). Other species with extensively-webbed feet include Chiropterotriton magnipes and Oedipina carablanca. Convergent and parallel evolutionary trends are observed also for elongate, fossorial forms among the tropical genera Lineatriton and Oedipina (Parra-Olea and Wake, 2001).
Characteristics
Detailed Characteristics of the Supergenus Bolitoglossa
Morphological characteristics detailed below are summarized from Lombard and Wake's (1986) phylogenetic analysis of major plethodontid lineages with special emphasis on evolution of feeding mechanisms. These characteristics are useful in combination for distinguishing bolitoglossine salamanders from other plethodontids, although the characteristics listed are not synapomorphies of the supergenus Bolitoglossa.
Tongue and Hyobranchial Apparatus
Members of the supergenus Bolitoglossa have freely projectile tongues (i.e., lack the genioglossus muscle and anterior connective tissue; Lombard and Wake, 1986). The hyobranchial skeleton lacks a urohyal, has an expanded basibranchial, and radii that are closely attached to and continuous with the basibranchial. The epibranchial is relatively longer than the basibranchial and first ceratobranchial. The second ceratobranchial is larger in diameter than the first ceratobranchial and constitutes the primary force-transmitting element in the movement of the tongue. The rectus cervicis profundis muscle is folded dorsally near its anterior end. The slip in the rectus cervicis superficialis has been lost. The omohydoideus, genioglossus, and circumglossus have been lost. The intraglossus is attached to the anterior end of basibranchial, lingual cartilage, or equivalent, or to the anterior end of the glossal ligament, ventral to the basibranchial. The basiradialis muscle may be present or absent. The hyoglossus muscle has a complete anterior section and posteriorly-oriented fibers in the posterior section. The suprapeduncularis muscle is present. The ramus hypoglossus bifurcates posteriorly, between the attachment of the first ceratobranchial to the basibranchial and to the epibranchial.
Epibranchial Number
Embryos have a single epibranchial.
Tail Autotomy
Cutaneous wound healing in the tail occurs, there are 2 caudosacral vertebrae, the first caudal vertebra is specialized, and tail breakage is localized.
Brain Stem Motor Column
There is only one class of brain stem motor column cells.
Jaws, Cranial Osteology and Structure of the Inner Ear
The bony shelf of the preorbital process is absent. A distinct lateral spur on the parietal is present. Premaxillary bones are relatively slender, and the intermaxillary gland is posterior to the pars dentalis. The periotic canal is straight or with a dorsal loop after it leaves the periotic cistern. Growth of otic semicircular ducts is nearly isometric.
Chromosome Number
The diploid number of chromosomes is 26.
Development
Development is direct.
Classification
The supergenus Bolitoglossa at present includes 13 genera (Wiens et al., 2007). Three genera are involved in a paraphyletic to polyphyletic relationship with Pseudoeurycea, and revisionary studies are in progress. The two species of Ixalotriton form a clade but their sister taxon is unclear. Wiens et al. (2007) found Parvimolge to be the sister taxon, but without strong statistical support. Parvimolge may be the sister taxon of Pseudoeurycea , Ixalotriton , Lineatriton. Lineatriton is diphyletic with respect to mtDNA and is deeply nested within Pseudoeurycea, close to species associated with P. leprosa. The diminutive Cryptotriton is at present improbably resolved as the sister taxon of all remaining members of the supergenus. Bolitoglossa, the largest genus, is comprised of 6 subgenera, based on mtDNA, all well-supported statistically (Parra-Olea et al., 2004).
Discussion of Phylogenetic Relationships
Phylogenetic relationships within genera of tropical bolitoglossines have been inferred primarily using mitochondrial DNA sequence data (e.g., García-París and Wake, 2000; García-París et al., 2000; Parra-Olea and Wake, 2001; Parra-Olea 2002, 2003; Parra-Olea et al. 2002, 2004; Wiens et al., 2007). Frost et al. (2006) used both mitochondrial and nuclear DNA sequence data in their higher-level analysis of amphibian relationships, which included 10 of 13 tropical bolitoglossine genera, but they used only data from GenBank for most of the species, and had nuclear data for only three species. Wiens et al. (2007) investigated relationships among genera with more comprehensive taxon sampling but using only mitochondrial DNA sequences.
The results of Wiens et al. (2007) and Frost et al. (2006) are generally concordant, placing Thorius sister to a clade comprising Bolitoglossa, Pseudoeurycea and related smaller genera nested within Pseudoeurycea (Ixalotriton, Lineatriton). Both Wiens et al. (2007) and Frost et al. (2006) recovered Oedipina, Nototriton, and Dendrotriton together in a basal clade. However, Wiens et al. (2007) placed Parvimolge with Pseudoeurycea while Frost et al. (2006) placed it with Bolitoglossa. Wiens et al. (2007) placed Cryptotriton as sister to all other tropical bolitoglossines, while Frost et al. (2006) placed Cryptotriton sister to Dendrotriton. Wiens et al. (2007) placed Nyctanolis sister to Dendrotriton, with these two sister to a clade containing Nototriton and Oedipina , Bradytriton.
References
Alberch, P. 1981. Convergence and parallelism in foot evolution in the neotropical salamander genus Bolitoglossa, I. Function. Evolution 35:84-100.
Frost, D. R., T. Grant, J. Faivovich, R. H. Bain, A. Haas, C. F. B. Haddad, R. O. De Sá, A. Channing, M. Wilkinson, S. C. Donnellan, C. J. Raxworthy, J. A. Campbell, B. L. Blotto, P. Moler, R. C. Drewes, R. A. Nussbaum, J. D. Lynch, D. M. Green, and W. C. Wheeler. 2006. The Amphibian Tree of Life. Bulletin of the American Museum of Natural History 297, 370 pp.
García-París, M., and D. B. Wake. 2000. Molecular phylogenetic analysis of relationships of the tropical salamander genera Oedipina and Nototriton, with descriptions of a new genus and three new species. Copeia 2000:42-70.
García-París, M., D. A., Parra-Olea, G., and Wake, D. B. 2000. Biodiversity of Costa Rican salamanders: implications of high levels of genetic differentiation and phylogeographic structure for species formation. Proceedings of the National Academy of Sciences USA 97:1640-1647.
Lombard, R. E. and D. B. Wake. 1986. Tongue evolution in the lungless salamanders, family Plethodontidae. IV. Phylogeny of plethodontid salamanders and the evolution of feeding dynamics. Systematic Zoology 35:532-551.
Parra-Olea, G. 2002. Molecular phylogenetic relationships of neotropical salamanders of the genus Pseudoeurycea. Molecular Phylogenetics and Evolution 22:234-246.
Parra-Olea, G. 2003. Phylogenetic relationships of the genus Chiropterotriton (Caudata: Plethodontidae) based on 16S ribosomal mtDNA. Canadian Journal of Zoology 81:2048-2060.
Parra-Olea, G., and D. B. Wake. 2001. Extreme morphological and ecological homoplasy in tropical salamanders. Proceedings of the National Academy of Sciences USA 98:7888-7891.
Parra-Olea, G., M. Garcia-Paris, and D. B. Wake. 2002. Phylogenetic relationships among the salamanders of the Bolitoglossa macrinii species group (Amphibia: Plethodontidae), with descriptions of two new species from Oaxaca (México). Journal of Herpetology 36:356-366.
Parra-Olea, G., M. Garcia-Paris, and D. B. Wake. 2004. Molecular diversification of salamanders of the tropical American genus Bolitoglossa (Caudata: Plethodontidae) and its evolutionary and biogegraphical implications. Biological Journal of the Linnean Society 81:325-346.
Wake, D. B. 1991. Homoplasy: the result of natural selection, or evidence of design limitations? American Naturalist 138:543-567.
Wake, D. B. and J. F. Lynch. 1976. The distribution, ecology and evolutionary history of plethodontid salamanders in tropical America. Natural History Museum of Los Angeles County Science Bulletin 25:1-65.
Wiens, J. J., G. Parra-Olea, M. García-París, and D. B. Wake. 2007. Phylogenetic history underlies elevational biodiversity patterns in tropical salamanders. Proceedings of the Royal Society B 274:919-928.
Title Illustrations

| Scientific Name | Bolitoglossa striatula |
|---|---|
| Location | Costa Rica |
| Specimen Condition | Live Specimen |
| Source | Bolitoglossaram |
| Source Collection | Flickr |
| Image Use |
 This media file is licensed under the Creative Commons Attribution-NonCommercial-ShareAlike License - Version 2.0. This media file is licensed under the Creative Commons Attribution-NonCommercial-ShareAlike License - Version 2.0.
|
| Copyright | © 2006 Jack Goldfarb |
About This Page
Tom Devitt

University of California, Berkeley, California, USA
David Wake

University of California, Berkeley, California, USA
Correspondence regarding this page should be directed to David Wake at
Page copyright © 2007 and David Wake
All Rights Reserved.
- First online 09 March 2007
- Content changed 09 March 2007
Citing this page:
Devitt, Tom and David Wake. 2007. supergenus Bolitoglossa. Version 09 March 2007 (under construction). http://tolweb.org/supergenus_Bolitoglossa/68802/2007.03.09 in The Tree of Life Web Project, http://tolweb.org/






 Go to quick links
Go to quick search
Go to navigation for this section of the ToL site
Go to detailed links for the ToL site
Go to quick links
Go to quick search
Go to navigation for this section of the ToL site
Go to detailed links for the ToL site